Prospective Breast Cancer Biobank
A population-based biobank in Western Norway with a long follow-up time.
Breast cancer is the most frequent cancer among women. New biomedical knowledge enables long-term monitoring of patients with a view to the effect of the treatment given today and the development of biomarkers that can alert earlier of relapse. PBCB follows breast cancer patients for 11 years to form a unique research foundation in this patient group.
In Norway, more than 3,000 women are affected by breast cancer every year and over 40,000 women today live after undergoing treatment with varying degrees of side effects. After treatment given in conjunction with surgery, some patients will receive additional treatment for several years (up to 10 years) after surgery. Despite relatively good prognosis for this patient group, some of them will experience both adverse reactions that inhibit quality of life and both early and later relapses. There is therefore a need to be able to monitor patients by liquid biopsies in order to optimise treatment, to reduce side effects and to detect relapses very early in order to get the correct and new treatment started early.
Since 2012, PBCB has collected liquid biopsies in the form of blood samples and urine samples together with clinical information and patient-reported data (questionnaires = PROM data) of almost 1200 patients who have undergone breast cancer surgery at Haukeland University Hospital (HUS) and Stavanger University Hospital (SUS). This collection enables several important types of studies on breast cancer in our region, and forms the basis for research collaboration nationally and internationally.
The research group at HUS
PBCB's research group at Haukeland University Hospital consists of researchers and clinicians from the hormone laboratory and the endocrine surgery.
The Hormone Laboratory's research group (https://www.uib.no/en/rg/hormonelaboratory) is led by professors Gunnar Mellgren and Jørn Sagen. The group conducts research on breast cancer with a focus on endocrine therapy and advanced drug analysis (mass spectrometry). In addition, the group is working on a number of projects focusing on obesity, nutrition and type 2 diabetes. The group is affiliated with the Mohn Nutrition Research Laboratory and the Department of Clinical Medicine (K2) at the University of Bergen.
The Department of Endocrinsurgery is headed by Dr. med. Turid Aas, and the department has played a key role in collecting patient samples and questionnaires for PBCB. This department is working on research projects that will map side effects and psychosocial challenges associated with breast cancer treatment.
| PhD candidate |
|
Title | Status | ||
| Thomas Helland, molekylærbiolog | Gunnar Mellgren, Håvards Søiland, Emiel Janssen | Tamoxifen in the treatment of luminal breast cancer –Implications of active metabolites on gene expression, side effects and clinical outcome | Defended 22.02.2019 | ||
| Kari Britt Hagen, sykepleier | Ragna Lind, Håvard Søiland | Defended 23.09.2019 | |||
| Magnus Hagland, lege | Håvard Søiland, Gunnar Mellgren, Thomas Helland | Juxta-tumoral adipose tissue…. |
|
More information about the various research projects can be found on the front page.
Research Group for Breast Cancer at SUS
The Research Group for Breast Cancer (FFB) focuses on the entire treatment course for breast cancer patients. The group is therefore composed across departmental boundaries and along the patient's pathway axis. FFB consists of molecular biologists, pathologists, radiologists, oncologists, breast and endocrine surgeons, plastic surgeons, nursing researchers, nurses, research coordinators, user representatives and representatives from the medical humanities field (cultural scientist). Together we create the pillars of the group's motto: From molecule to fully human. Our vision is to reduce over- and under-treatment of breast cancer patients, as well as improve their quality of life by improving current diagnostics and follow-up through combined basic and translational research. This group was started by professor and surgeon Håvard Søiland and is now led by Kristin Jonsdottir.
At the Department of Pathology, several studies have been conducted with retrospectives based on previously archived tumour tissue collected in the period 1989-2004 from breast cancer patients. These studies are a combination of classical pathology, molecular biology, quantitative immunohistochemistry and digital pathology. The overall purpose of the studies is to improve current diagnostics.
In the Department of Blood and Cancer, translational breast cancer research has long been focused on the development of methods for detecting disease recurrence earlier than with current technology. Detection of micrometastases in bone marrow in patients with breast cancer (early stage) has long been the research focus here. In recent years, however, the focus has shifted more to investigating the importance of circulating tumour cells (CTCs) and cell-free tumour DNA (ctDNA) from plasma as markers for predicting disease recurrence and as a tool for measuring treatment efficacy.
https://red-helse-stavanger.hn.nhn.no/seksjon/forskning/Sider/pbcb_samarbeid.aspx
The goal is to include 1200 patients who are diagnosed with breast cancer and who are treated at Helse Bergen or Helse Stavanger, and follow them 11 years after treatment. Blood and urine samples are collected annually, as well as questionnaires. In Stavanger, blood and urine samples are also collected semi-annually.
Exclusion criteria: Stavanger does not include patients undergoing neoadjuvant therapy prior to surgery. Patients must be able to complete Norwegian questionnaires. The patient must not have had breast cancer before they are included in the study. The patient must be of legal age, over 18 years of age.
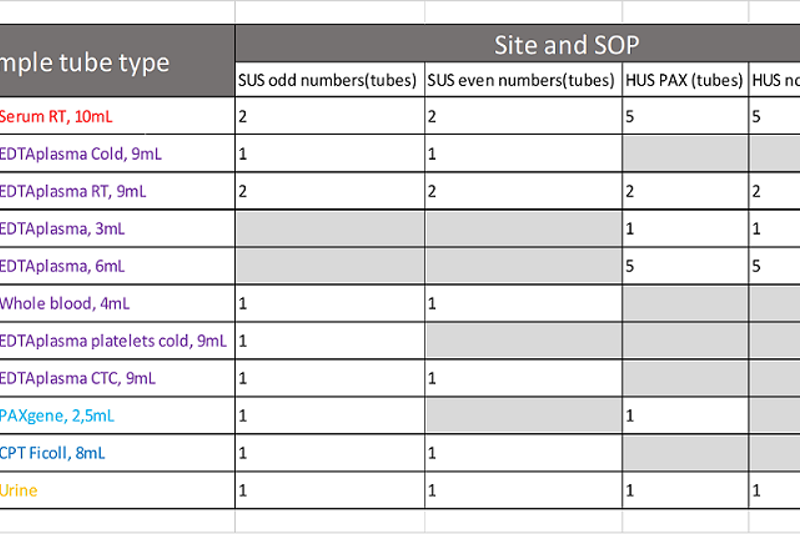
Stardard operating procedure
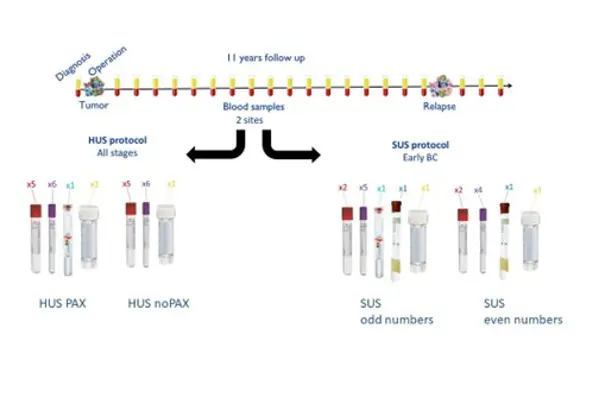
Sample kit
Questionnaire (PROM)
|
Name |
Description |
|
EORTC-QLQ-C30 EORTC-QLQ-BR23 |
Quality of life questionnaire. The questionnaires consist of 53 questions about daily activities, physical and mental. |
|
The Mishel Uncertainty in Illness Scale (MUIS) |
4 statements that map how information about illness and treatment has been perceived/understood |
|
Hospital Anxiety and Depression Scale (HAD) |
14 questions of which 7 assess anxiety and 7 depression. |
|
Functional Assessment of Cancer Therapy-Fatigue (FACT-F) |
13 questions about fatigue in the past 7 days |
|
Functional Assessment of Cancer Therapy-Endocrine Scale (FACT-ES) |
56 questions about how patients have felt in the last 7 days. The questions include physical and mental conditions as well as side effects of endocrine treatment. |
|
Functional Assessment of Cancer Therapy-Breast (FACT-B) |
44 questions about quality of life in breast cancer patients |
|
Fatigue Severity Scale (FSS) |
9 questions about how one has experienced feelings of tiredness and exhaustion in the past week. |
|
fatigue Visual Analog Skala (fVAS) |
VAS is a line where on the far left of the line it is 0 = "no problems with tiredness and exhaustion" and on the far right is 100 = "as much tiredness and exhaustion as it is possible to have. |
|
Food Form |
Questionnaire avoiding any of 36 different foods with response alternatives "Yes", "No", "Partial". Seven additional questions about the use of milk and milk products, and one about the use of alcohol. |
|
Questions about joint pain |
3 questions about joint pain with answer option yes/no and grading of morning stiffness in the joints and any muscle pain. |
|
Irritable bowel syndrome (Rome III, short version) |
5 questions that map irritable bowel syndrome (IBS) and 5 additional questions that characterize the symptoms |
|
Compliance |
A modified version of the Morisky-8 item Medication Adherence Scale (MMAS) which also contains 2 statements about "belief in the drug" |
|
Subjektive Helseplager (Subjective Health Complaints, SHC). |
The questionnaire consists of 29 questions about subjective, somatic and psychosocial problems during the last 30 days. The SHC has four subgroups: musculoskeletal pain, pseudoneurology, gastrointestinal distress, allergies and colds |
|
Second |
Questions about side effects from the use of tamoxifen and aromatase inhibitors. |
PBCB has appointed a steering committeeto ensure that the biological material and associated data are managed in accordance with applicable laws, regulations and internal instructions for research. The committee processes applications from research projects for access to and use of biological material or results from analyses, and facilitates interaction with external institutions to the extent warranted by the activities of PBCB. The committee is responsible for ensuring that the interests of the participants are safeguarded.
The steering committee consists of the following members:
| Role | Name | Position |
| Leader | Prof.dr.med. Gunnar Mellgren | Clinic Director Laboratory Clinic, HUS |
| Styremedlem | Prof.dr.med. Håvard Søiland | Consultant Breast- and Endocrinology Outpatient clinic, SUS |
| Styremedlem | Prof.dr.med. Jørn Vegard Sagen | Head of Department Hormone Laboratory, HUS |
| Styremedlem | Dr. med. Turid Aas | Head of Department of Surgical Clinic, HUS |
| Styremedlem | Dr. med. Tone Hoel Lende | Head of Department Breast- and Endocrinology Outpatient clinic, SUS |
| Styremedlem | Prof. Emiel Janssen | Head of Section of Quantitative and Molecular Pathology, SUS Head of the Laboratory of Molecular Biology, SUS |
| Styremedlem | Ann Cathrine Kroksveen, Ph.D. | Head of Section Biobank Haukeland, HUS |
| Styremedlem | Kristin Jonsdottir, Ph.D. | Advisor and Biobank coordinator, SUS |
Submitting biological material to PBCB is voluntary and requires consent. It will have no bearing on treatment if one chooses not to provide a sample, or if one later wishes to withdraw. Relevant participants to the biobank are asked during a consultation at the hospital if they would like to contribute biological material and data to PBCB. Participants receive oral information about the biobank, as well as an information letter. Participants agree that the material and data may be used for future research in breast cancer. New projects that will use material and data from PBCB will be assessed by a regional committee for medical and health research ethics (REK). Such a committee will approve the use of samples/data in individual studies if they believe that the study is covered by the consent given by the trial participant. The biological material and metadata will be stored permanently in the biobank. All other information will be related to specified research projects. Such information will be deleted/anonymized when the various projects have been completed. It will not be possible to identify the participants in the results of the various studies when these are published. The participants may withdraw their consent at any time without this affecting the treatment provided at the hospital. If the participant withdraws from the study, the participant can choose whether already collected biological material can be used for further research or whether this material should be destroyed.
Folke Hermansen Fund
Breast Cancer Association
Western Norway Regional Health Authority
Inge Steensland Foundation
For participants:
Research Coordinator Stavanger, email: pbcb@sus.no or phone: 51514434
Research Coordinator Bergen, Nurse Sissel Velve: sissel.velve@helse-bergen.no
For researchers:
Professor Gunnar Mellgren, Regional Leader of PBCB: gunnar.mellgren@helse-bergen.no
Professor Håvard Søiland, Head of PBCB in Stavanger: havard.soiland@sus.no
Biobank coordinator Stavanger, Kristin Jonsdottir: kristin.jonsdottir@sus.no
Biobank manager Biobank Haukeland Ann Cathrine Kroksveen: ann.cathrine.kroksveen@helse-bergen.no